The founders envisioned having a team of individuals committed to develop innovative ideas in ACT diagnosis and treatment
The ICPACT founders agree to participate in collaborative research efforts including the development and validation of methods and projects
The Founders: Ana Paula Percicote, Bonald Cavalcante de Figueiredo, Camila M. Daiggi, Carmem M. C. M. Fiori, Caroline Brunetto, Eliana N. N. Caran, Emília Modolo Pinto, Enzo Lalli, Gabriela Caus Fernandes Luiz Canali, Gerard P. Zambetti, Humberto Cereser Ibañez, José Alexandre Marzagão Barbuto, Karla Emilia de Sá Rodrigues, Lauro Mera Souza, Mara Albonei Dudeque Pianovski, Maria José Mastellaro, Pablo Santiago, Patricia Brandalise, Raul Correa Ribeiro, Simoni Abib, Silvia Brandalise, Tatiana El Jaick B. Costa, Verena Wiegering and Vilani Kremer.
ENROLLED ICPACT MEMBERS (since March 28, 2024):
NEW MEMBERS ARE WELCOME!
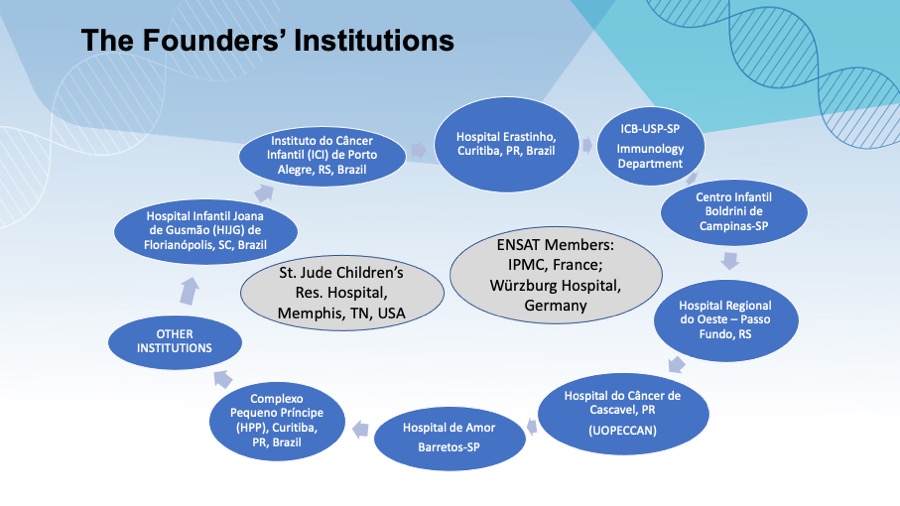
Hospital Erastinho, Curitiba, PR, Brazil
Mara Albonei Dudeque Pianovski
Robson de Castro Coelho
Sérgio Ioshii
Vilani Kremer
Instituto do Câncer Infantil, Porto Alegre, RS, Brazil
Algemir Brunetto
Caroline Brunetto de Farias
Julie Cerutti
Centro Infantil Boldrini, Campinas, SP, Brazil
Camila M. Daiggi
Caroline Poli Belluco
Izilda Aparecida Cardinalli
Iva Loureiro Hoffmann
Luiz Henrique Pereira
Marcia Alessandra Cavalaro Pereira da Silva
Renato Luis Giampietro Bonfá
Silvia Regina Brandalise
Suelen Nascimento Meirelles
Hospital Infantojuvenil de Barretos, Hospital do Amor, Barretos, SP, Brazil
Karla Emília de Sá Rodrigues
Luiz Fernando Lopes
Thais Junqueira
Hospital Joana de Gusmão, Florianópolis, SC, Brazil
Denise Bousfield da Silva
Tatiana El-Jaick Bonifacio Costa
Hospital São Vicente de Paulo, Passo Fundo, RS, Brazil
Pablo Santiago
Hospital Infantil de Joiville, Joinville, SC, Brazil
Patricia Brandalise
Department of Immunology, Instituto de Ciências Biológicas (ICB), Universidade de São Paulo, SP, Brazil
José Alexandre Marzagão Barbuto
Instituto de Oncologia Pediátrica – GRAACC (Grupo de Apoio ao Adolescente e a Criança com Câncer), Universidade Federal de São Paulo, São Paulo, SP, Brazil
Eliana Maria Monteiro Caran
Simone de Campos Vieira Abib
Endocrinology Division, University of Michigan. Ann Arbor, United States
Antonio Marcondes Lerario
União Oeste Paranaense de Estudos e Combate ao Câncer – UOPECANN, PR, Brazil
Carmem Maria Costa Mendonça Fiori
Hospital de Pediatría Garrahan, UBA, Argentina
Alicia Belgorosky
Daniela Fortunati
Gabriela Guercio
Laura Laura Galluzzo Mutti
María Celeste Mattone
Maria Sonia Baquedano
Paula Flores
Silvia Gil
University of Pennsylvania Perelman School of Medicine, Philadelphia PA, USA
Kotaro Sasaki
University hospital Wuerzburg, Department of Pediatrics, Würzburg, Germany
Verena Wiegering
Maria Riedmeier
Institut de Pharmacologie Moléculaire et Cellulaire, Valbonne, France
Carmen Ruggiero
Enzo Lalli
Mabrouka Doghman
St. Jude Children’s Research Hospital, Memphis, TN, EUA
Emília Modolo Pinto
Gerard Paul Zambetti
Raul Correa Ribeiro
Hospital e Instituto de Pesquisa Pequeno Príncipe
Ana Paula Kuczynski Pedro Bom
Ana Paula Percicote
Bonald Cavalcante de Figueiredo
Edna K. Carboni
Flora Mitie Watanabe
Gabriela Caus Fernandes Luiz Canali
Humberto Cereser Ibañez
Lauro de Souza
Leila Grisa
Silvio Gilberto Andrade Avilla
Our mission
“To construct the future with the lowest mortality rates for pediatric adrenocortical carcinoma”
Overall objectives
- Decrease the number of cases presenting with advanced-stage disease
- Create a clinical-relevant risk classification of pediatric ACT
- Find the best parameters to monitor Mitotane effects (metabolomics)
- Develop efficient therapeutic protocols
- Support early onset stage I diagnosis
Chairperson

- Pediatric Oncologist, Centro Infantil de Investigações Hematológicas Dr. Domingos A. Boldrini
- From November, 2024 to November, 2025: Camila Maia Martin Daiggit, MD.
The next volunteer to become chairperson (Emilia M. Pinto) will be in charge of organizing the next ICPACT International Workshop.
The responsibilities of the chairperson (with support of all members) shall be:
- To propose and facilitate collaborative treatment protocols and projects on PACT;
- To oversee the progress of the collaborative projects undertaken by ICPACT;
- To seek funds on behalf of the ICPACT Workshops;
- To organize on-line regular meetings of the Plenum and at those meetings, to inform the Plenum of all significant ICPACT activities;
- To act on behalf of the Plenum in the periods between meetings of the Plenum.
Membership of the Plenum
Additional members and Institutions can also be appointed by any Founding Members. Membership in the Plenum carries with it acceptance of the following responsibilities:
- Attendance at regular meetings;
- Ethical use of information and biological material obtained through the ICPACT;
- Acceptance to submit scientific data relating to current studies of the ICPACT
- Adherence to the proprietary rules established by the Plenum regarding biological samples and clinical information, results of clinical or laboratory studies, and authorship;
- Adherence to the Good Clinical Practice (GCP) and national laws relating to the conduct and reporting of results of clinical trials.
The Chairperson will have the support of seven chairpersons:
- Laboratory and Diagnostic Guidelines
- Repository of Biological Materials or Patient/Research Subject Data
- Web Registration and Auditing
- Clinical studies
- Chemotherapy and mitotane
- Immunotherapy
- Screening and Surveillance
In order to provide the foundation for future research, participants shall utilize a standard method for collecting and storing materials and data. Blood/plasma samples, ACT samples, and data relating to specific patients or research subjects will be collected by local researchers, according to the protocols implemented and the informed consent gained from patients or subjects.
Previous Chairpersons:
- Bonald C. Figueiredo, MD, PhD, Founder (2023-2024).
Authorship
- All publications, proposed within the ICPACT, shall carry under the title the following citation: “A report from the International Consortium of Pediatric Adrenocortical Tumors (ICPACT)”;
- All articles proposed within the ICPACT shall be submitted for publication if approved in advance by the Steering Committee;
- Authorship order shall be previously established and will be according to extent of responsibility and participation of each ICPACT Member.


