Metabolomics of mitotane: metabolites levels that may predict clinical outcome using 2D Chromatography
Because of restrictive regulations by ANVISA (the Brazilian “FDA”) it is extremely difficult to receive frozen samples from foreign countries, and we prefer to receive plasma or serum only from Brazilian Institutions
There is widespread agreement that the management of ACC patients with persistent or recurrent disease could be benefited by better control of mitotane treatment, specially regarding metabolomics, which includes uncertainties in the therapeutic window (including mitotane and its NOT YET PROPERLY EXAMINED 3 main metabolites, 2 DDA and 1 DDE).
Here the consortium may be benefited with unlimited number of tests of ACT patient samples to be analyzed by Dr. Lauro M. Souza (Pequeno Príncipe Research Institute), and frozen serum samples may be sent and communicated directly to his Whatsapp (+55 41 99229 0618). The idea is to define levels and outcome according to timing, age, dose, steroid levels, persistent microscopic/macroscopic lesion, etc).
Participants will be coauthors of this future article (next 18 months):
MITOTANE METABOLOMICS USING 2D Chormatography
Simple Mitotane TDM (Therapeutic Drug Monitoring over the time)
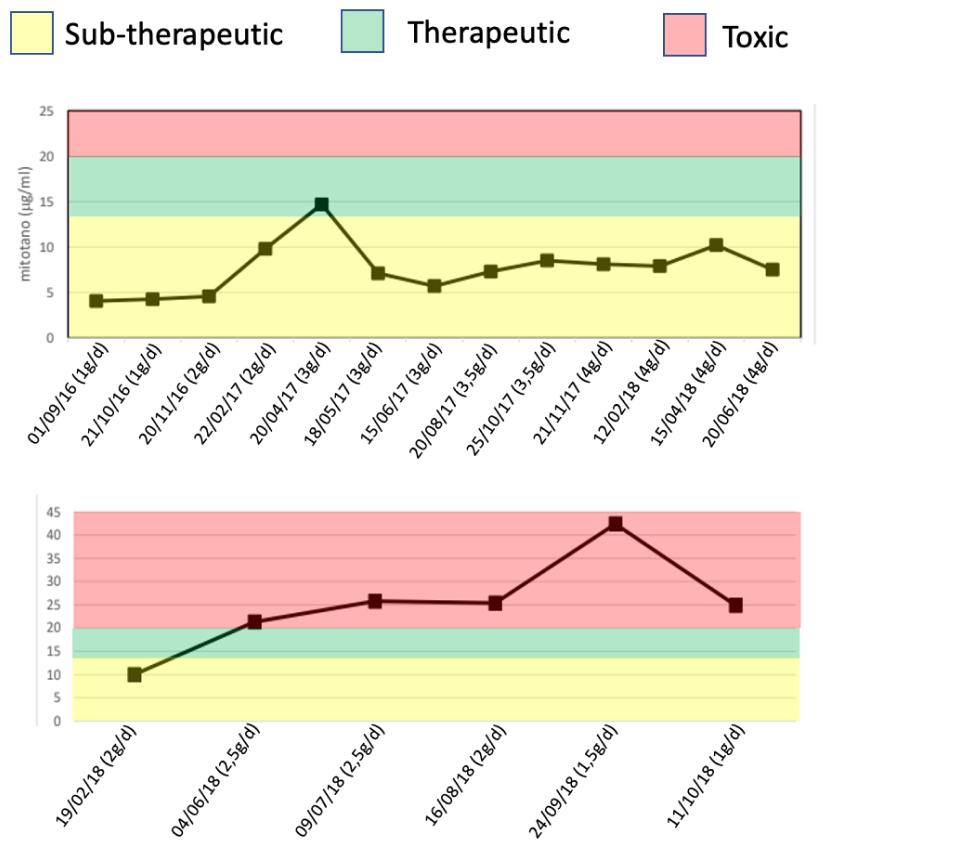
Two opposite profiles:
(a) Subtherapeutic in patient 1, despite increased daily dose;
(b) Supratherapeutic (toxic) in patient 2, despite decreased daily dose.
We believe that these differences need to be investigated together with all metabolites using 2D chromatography
Two metabolic profiles in R1 children
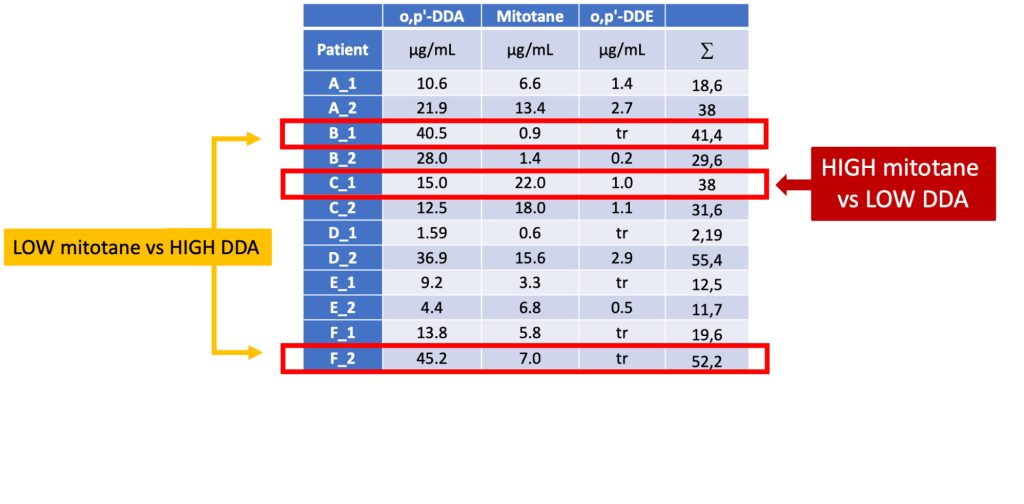
Preliminary data (Stadler et al., 2023) to illustrate the need for future studies associated with outcome and side effects
Plasma from R1 and R2 pACT patients on mitotane
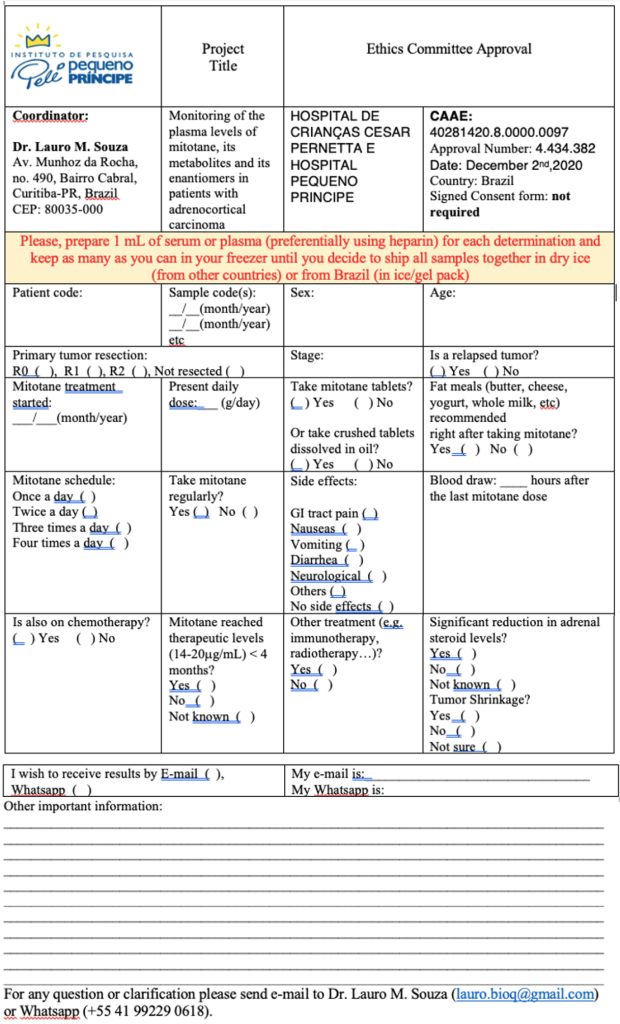
Sample Analysis Form
This is a Research Project, entitled “investigation of mitotane and its metabolites in sera/plasma of ACT patients”, approved by the Hospital Pequeno Príncipe Ethics Committee (EC). If you decide to request analyses for your patients you will need previous approval from your Institution’s EC (or IRB).
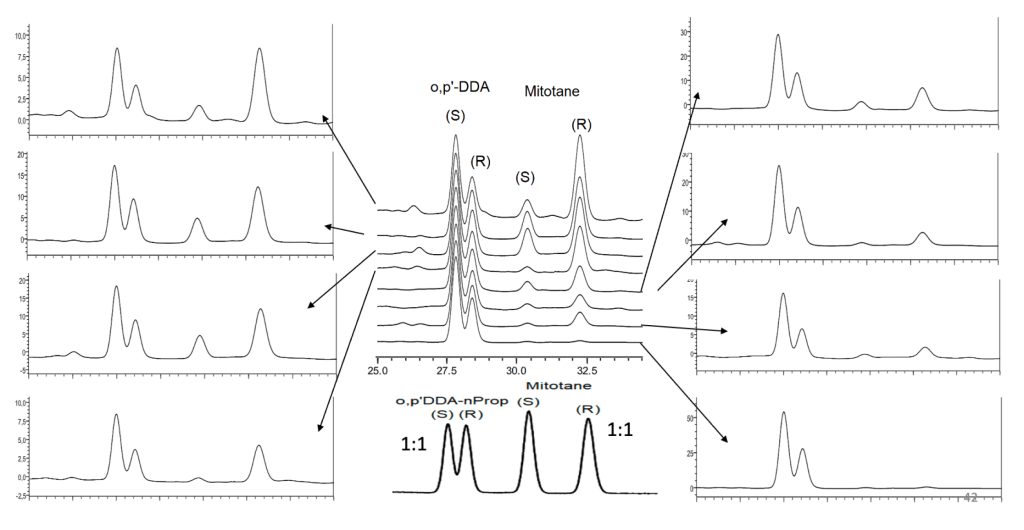
Reported results/sample: mitotane (R & S), o,p’-DDA (R & S) and o,p’-DDE
Method: Liquid Chromatography
Preferential sample and volume: at least 1 mL of plasma in heparinized tubes sent in dry ice (if you are in another country) or in gel pack (if you are in Brazil) to:
Instituto de Pesquisa Pelé Pequeno Príncipe
Av. Munhoz da Rocha, no. 490
Bairro Cabral, Curitiba-PR
CEP: 80035-000
Chromatography Laboratory
Dr. Lauro M. Souza and Alan A. Veiga
Considering that transport is expensive, you may accumulate samples and send later to Curitiba.


