New anti-steroidogenic drugs: osilodrostat (Recordati) and the Orphagen new drug (no conflict of interest).
It is clear that we cannot design any immunotherapy clinical trial without full inhibition of the steroidogenesis. The successful cases treated with checkpoint inhibitors (nivolumab and Ipilimumab) were in patients with nonfunctional ACC (cases presented at Varandas Workshop).

Two metabolic profiles in R1 children
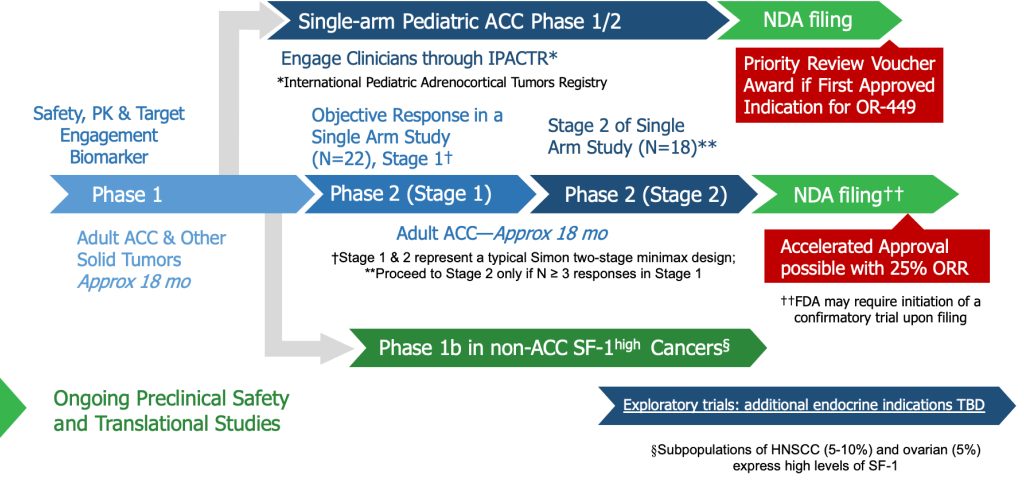
Immunotherapy
Inhibitory of checkpoints: Nivolumab and Ipilimumab
Unfortunately, we do not have yet the possibility to get these expensive antibodies from the Public System (in Brazil called SUS) because we need more scientific data to justify this for ACC patients. However, it is in our mind to support fundraising for use of this consortium. It is expected each Hospital could start fundraising campaigns to import Nivolumab and Ipilimumab.
It is expected that each Hospital could start fundraising campaigns to import nivolumab and Ipilimumab, however we could also (fostered by SOBOPE) negotiate directly with the industry.
Inhibition of checkpoints (without hipercortisolism): Nivolumab + Ipilimumab or Pembrolizumab
In Europe, immunotherapy is NOT reimbursed for either pediatric or adult ACC. There is an ongoing French trial (Spencer Trial, PI Eric Baudin) with Nivolumab + adjuvant vaccine based on microbiota-derived peptides in patients with advanced ACC (only for adult patients and patients are first selected on HLA-I haplotype).
MD Anderson Cancer Center, Muhammad Habra utilizes immunotherapy (Pembrolizumab) for his patients with advanced ACC.
In the USA, immunotherapy is NOT FDA-approved for ACC, but they have the possibility to prescribe it. We as a consortium or Raul could ask Habra?
Finally, we could ask Bristol-Meyer for a, single-patient, compassionate use. They are usually quite restrictive, but never say never!
The Xenograft model (mouse with pediatric ACC)
- T Lymphocytes-CD8+
- T Lymphocytes-CD8+ and hybrid cells
- Only hybrid cells
Pequeno Principe Therapeutic Vaccine:
The hybrid cell (Dendritic cell +ACC cell) incubated with T lymphocytes
B7-H3 CAR-T Cells for ACC:
Xenograft Mouse Models (concluded study) and ongoing Clinical Trial (Phase 1) not for international patients
Promising preliminary data for B7-H3 CAR T cell therapy in pediatric solid tumors, including the SJ-ACC xenograft models. Single dose also eliminated SJACC4 and MAST449A (not SJACC3, low expression of B7-H3).
3CAR: B7-H3-specific CAR T-cell Therapy for Children and Young Adults with Solid Tumors
Chris DeRenzo and Rebecca Epperly (St. Jude BMT & Cell Therapy)
3CAR is a Phase 1 clinical trial evaluating the safety and efficacy of autologous B7-H3-specific CAR T-cell therapy for children and young adults with relapsed or refractory solid tumors.
Primary objective
To determine the safety and maximum tolerated dose of one intravenous infusion of autologous, B7-H3-CAR T cells in patients 21 years old and younger with relapsed or refractory B7-H3-positive solid umors after lymphodepleting chemotherapy
Secondary objective
To evaluate the antitumor activity of B7-H3-CAR T cells
Inclusion criteria:
21 yrs or younger; Relapsed or refractory B7-H3+ solid tumor; Measurable disease and Adequate heart, lung, liver, kidney and bone marrow function

